Neonatologists Demonstrate Role of Placenta in Neurologic Development
The role of the placenta as a connection between mother and baby to ferry oxygen and nutrients, filter toxins, mingle maternal and fetal immune systems, and other functions is well known. In a new study, NewYork-Presbyterian/
Fetus in the womb with placenta
Led by Anna Penn, MD, PhD, Chief of Neonatology at NewYork-Presbyterian/

Anna Penn, MD, PhD
ALLO is generated in the placenta from progesterone. ALLO levels rise significantly late in gestation, so prematurity can deprive a developing fetus from its benefits. "A preterm infant can lose up to 16 weeks of exposure to ALLO, and that's a huge loss," says Dr. Penn.
Children born prematurely are at increased risk of cognitive difficulties in areas such as perceptual-motor function, learning, memory, attention, and social cognition. Epidemiological studies have shown that premature infants have an elevated risk of neurodevelopmental disorders such as cerebral palsy, ASD, and schizophrenia.
Dr. Penn has dubbed the study of the placenta’s role in protecting and shaping the fetal brain "neuroplacentology." As a neonatologist with a PhD in developmental neuroscience, she became very interested in sex differences in outcomes in preterm infants, with males being at a much higher risk for neurodevelopmental impairment. She began designing studies to look at how the development of the cerebellum in premature babies—an understudied part of the brain that has been linked to autism—might contribute to long-term impairment.
Neuroplacentology: the study of the role of the placenta in protecting and shaping the fetal brain
"When I started my lab, I explored the impact of sex hormones on the cerebellum. It became clear that we needed a way to study the placenta, because it is the neuroendocrine organ that develops first," Dr. Penn explained. She and her lab team developed animal models to explore this area of study and detected a remarkable sexual dimorphism related to the presence or absence of ALLO.
ALLO modulates a GABA receptor which is involved in the regulation of many neurodevelopmental processes including neurogenesis, cell survival, cell migration, and synaptogenesis. GABA has been proposed as a pivotal factor underlying the differences in neurodevelopment in premature males versus females. ALLO also reduces anxiety and inflammation in pregnant women, but it is not clear why it is produced at much higher levels in the latter half of gestation.
In their 2021 study published in Nature Neuroscience, scientists created a mouse model in which progesterone could not be converted into ALLO. Claire-Marie Vacher, PhD, Assistant Professor in the Department of Pediatrics at NewYork-Presbyterian/
By giving a single intraperitoneal injection of ALLO to the dams late in pregnancy, the investigators were able to shift the behavior of the formerly ALLO-deficient males back into the normal range. "Our data were the first strong example of a hormone that alters late fetal neurodevelopment in such a striking way," Dr. Penn noted.
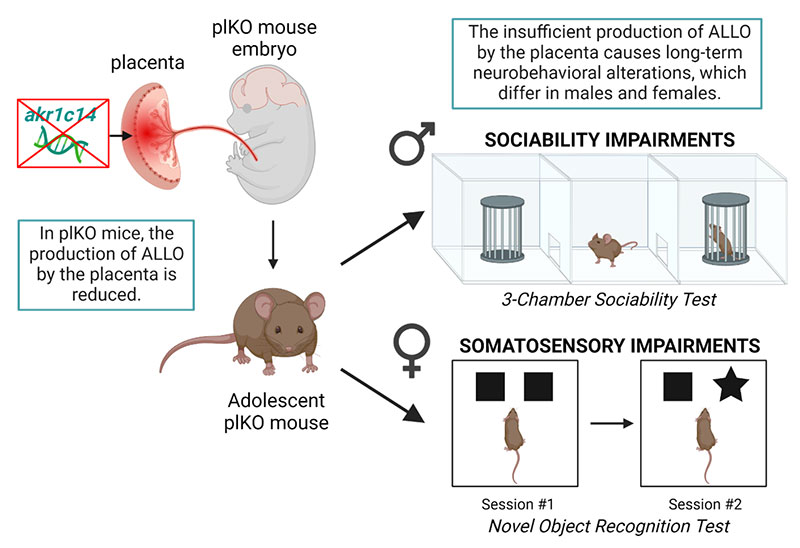
Long-term effect of placental ALLO insufficiency on behavior. Created with BioRender.com.
In the 2022 Frontiers in Endocrinology study, she and her fellow investigators reported that female mice lacking sufficient ALLO experienced alterations in the development of the sensory cortex—including a reduction in pyramidal neuron density, as well as somatosensory behavioral deficits. Deficiencies in somatosensory processing have also been observed in survivors of preterm births, impairing their social behaviors, motor skills, and ability to complete daily tasks. Reduced neuronal connectivity has also been associated with ADHD as well as ASD in premature infants.
“Our data were the first strong example of a hormone that alters late fetal neurodevelopment in such a striking way.” — Dr. Anna Penn
Dr. Penn and her team are continuing to focus on sex-linked differences in brain development, particularly the role of microglia, which are involved in neural pruning and the response to inflammation. Prior studies in humans have shown that the presence of inflammation during pregnancy, such as chorioamnionitis (infection of the placenta and amniotic fluid) and even the chronic inflammation of obesity, may alter fetal brain development. They are also very interested in other placental hormones that may impact neurodevelopment and are conducting a large-scale assessment of all of the related gene expression data from mice and humans that have been published.
The finding that injection of ALLO in the mouse model improved behavior in male offspring may lead to therapeutic strategies that could prevent devastating neurodevelopmental disorders linked to loss of placental function due to prematurity or placental insufficiency. "Giving a mother betamethasone 24 hours prior to a preterm delivery significantly improves preterm survival and outcomes," noted Dr. Penn. "If we can design a single-dose treatment that would have an impact on neurodevelopment, that would be our ultimate goal. But we're not there yet."
"Even though it's only used for up to 9 months, the placenta is a key part of the developing fetus—not just serving as an exchange with the mother and therefore the outside world, but also as a functioning fetal organ in and of itself with a long-term impact on how babies develop later in life," concluded Dr. Penn. "We should stop throwing it away and pay more attention to how it is contributing and functioning and what it can tell us about kids who are at risk for neurodevelopmental deficits."
Read More
Vacher C-M, Lacaille H, O'Reilly JJ, Salzbank J, Bakalar D, Sebaoui S, Liere P, Clarkson-Paredes C, Sasaki T, Sathyanesan A, Kratimenos P, Ellegood J, Lerch JP, Imamura Y, Popratiloff A, Hashimoto-Torii K, Gallo V, Schumacher M, and Penn A. Placental Endocrine Function Shapes Cerebellar Development and Social Behavior. Nature Neuroscience. 2021;24:1392–1401.
Bakalar D, O'Reilly JJ, Lacaille H, Salzbank J, Ellegood J, Lerch JP, Sasaki T, Imamura Y, Hashimoto-Torii K, Vacher C-M, and Penn A. Lack of Placental Neurosteroid Alters Cortical Development and Female Somatosensory Function. Frontiers in Endocrinology. Oct 13, 2022.
For More Information
Dr. Anna Penn
NewYork-Presbyterian
Advances in Pediatrics
Read more about our latest clinical advances.


