In March 2024, the U.S. Food and Drug Administration approved a paclitaxel-coated balloon for the treatment of coronary in-stent restenosis (ISR). The approval came at the same time as publication of the results of the AGENT IDE Trial, the largest randomized clinical trial and first-of-its-kind to date in the United States looking at the safety and efficacy of the drug-coated balloon catheter versus an uncoated balloon for ISR.
Ajay Kirtane, MD, Director of the Interventional Cardiovascular Care program at NewYork-Presbyterian/Columbia, served as study chairman and was the senior author on the paper published in JAMA. Below, Dr. Kirtane shares his perspective on the approval of this novel device:
As an interventional cardiologist, one of the biggest gaps that we’ve had in the coronary space in the United States was the lack of a drug-coated balloon to be able to treat patients with ISR. Traditionally when patients have stents that restenose, they’re treated with another stent. But that means leaving behind another layer of metal within existing stents, and we generally don’t want to put more and more layers of metal inside an artery, especially if we can avoid it. In some cases, we have had to use conventional balloons or radiation therapy, or even bypass surgery, especially in patients with multiple layers of stents. But we lacked a drug-coated balloon that could treat ISR, and that’s why we designed the AGENT IDE study – to show the safety and efficacy of a paclitaxel-coated balloon versus a conventional balloon for patients with ISR.
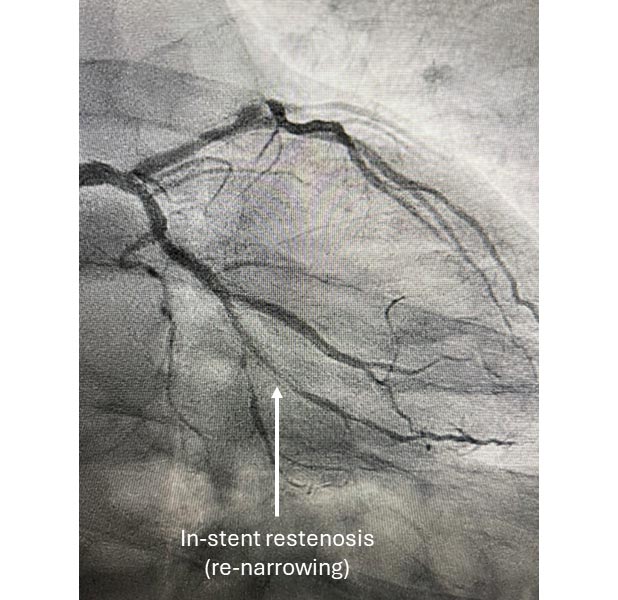
Angiogram showing coronary in-stent restenosis
We were amazed at how quickly the trial enrolled – it was one of the fastest enrolling trials I’ve ever been a part of – which speaks to the fact that this is truly an unmet need for patients and interventional cardiologists. For example, at NewYork-Presbyterian/Columbia, we were actively treating patients within the study and there were many who had limited treatment options, but now had an option with this drug-coated balloon. The results from the AGENT IDE trial (now published in JAMA) clearly show that the drug-coated balloon was superior to conventional balloon angioplasty for patients with ISR. To see the trial come to fruition and ultimately lead to a device approval means that the commitments each and every one of those individual patients made will now serve to help many other patients around the country. For me personally, being able to be so integrally involved in the study of this technology that can help so many but also ensuring it is safe for patients is also very gratifying.
While the device is now approved for use, it’s important to note that this drug-eluting balloon is not a cure for the problem of a restenosed stent. The most important way to prevent a stent from narrowing is to implant it with excellent technique the first time around. When you’re treating a stent that has restenosed, you really need to make sure that you optimize the stent dimensions before you use decide to use a drug-coated balloon because there’s no substitute for good technique. That will always be the optimal approach, however I think it’s incredibly important that we, as physicians, keep educating ourselves and making sure that we are doing angioplasty procedures with the most modern-day techniques and also using the most current devices to get the best outcomes for patients.
The approval of this device will change the standard of care for patients with ISR; however, it won’t be the go-to approach for all patients. This is just another tool in our arsenal that we can use when treating these patients. In some cases it may allow patients to avoid open heart surgery for example. That’s a welcome benefit for patients, but it is important to evaluate each case individually so that all options are on the table for our patients.
This approval is an important step for the treatment of ISR, though we must remain committed to continuously figuring out better ways to use these devices in the future.



