BRCA1 mutations dramatically increase the lifetime risk of breast, ovarian, pancreatic, and other cancers, yet most BRCA1-positive patients are not identified until after they have been diagnosed with cancer. At prenatal visits, women often undergo obstetrical prenatal carrier screening that can identify hundreds of genetic mutations which can be passed on to offspring and results in an inherited condition — however, BRCA1 and other autosomal dominant mutations are not included on these screening panels.
Recognizing a window of opportunity to identify BRCA1-positive women at a juncture when screening and preventive strategies can reduce morbidity and mortality, Shayan M. Dioun, MD, a gynecologic oncologist at NewYork-Presbyterian and Columbia, Melissa K. Frey, MD, a gynecologic oncologist and director of the Genetics and Personalized Cancer Prevention Program at NewYork-Presbyterian and Weill Cornell Medicine, and other researchers developed an innovative model that demonstrated the cost and clinical effectiveness of BRCA1 testing at the time of prenatal carrier screening. Below, Dr. Dioun and Dr. Frey discuss the findings and the potential of this approach to reduce disease and save lives.
A Novel Strategy to Identify At-Risk Women
Dr. Frey: This study was sparked by our efforts to expand our patients’ access to genetic testing. When we identify someone with a BRCA1 mutation it is usually in the setting of a breast or ovarian cancer diagnosis. As gynecologic oncologists, we see the entire continuum of women’s health care. Once a woman has cancer we have lost our opportunity for prevention. This perspective led us to ask, “How can we identify BRCA carriers before a cancer diagnosis so that we can prevent cancer in the first place?”
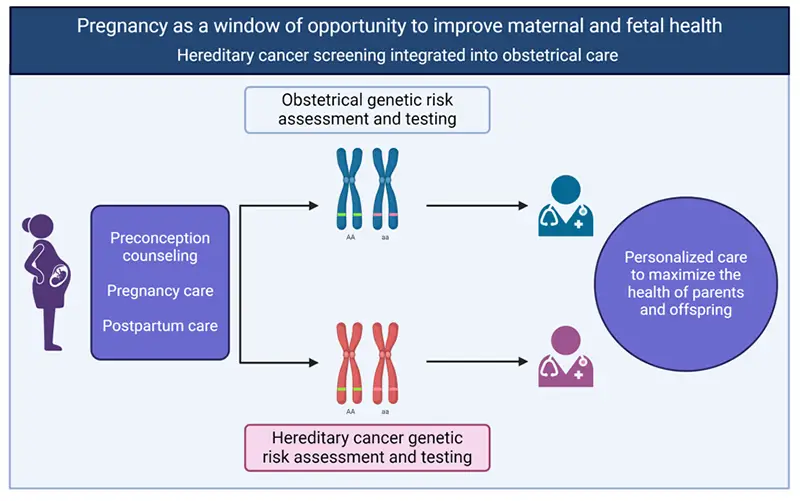
Adding BRCA1 to prenatal carrier screening may offer a window of opportunity to improve health for at-risk women. Created with Biorender.
Pregnancy may be an ideal time to offer cancer genetic screening to women. Pregnancy is a window of opportunity not only to improve fetal health with genetic risk assessment and testing, but also to improve maternal health through hereditary cancer genetic risk assessment and testing. This type of personalized care maximizes the health of both the mother and child.
By adding the BRCA1 test to prenatal carrier screening in our model, 3,716 patients with a BRCA1 mutation were identified who otherwise would not have known about their mutation.
— Dr. Shayan Dioun
Dr. Dioun: To gain acceptance of this idea, we wanted to address the common barriers to BRCA1 testing at the time of prenatal carrier screening. One issue that often comes up is the cost and time that providers would have to spend to counsel patients and send in the test. One goal of our study was to tackle these concerns.
Research Methods
Dr. Dioun: Our study simulated the clinical trajectory of a hypothetical cohort made up of more than 1.4 million pregnant patients who could receive BRCA1 testing if it were added to their prenatal carrier screening. Our model tracked patients starting at age 33 and followed them until age 80. For BRCA1-positive patients, our model simulated recommended breast cancer screening imaging and risk-reducing surgical interventions.
To estimate the cost effectiveness, we created a Markov model for BRCA1 testing at the time of prenatal carrier screening. The model’s probabilities, cost, and utility values were derived from previously published literature. We were fortunate to be able to collaborate across Columbia and Weill Cornell Medicine, drawing from experts on both campuses who excel in the fields of cost-effectiveness analysis and clinical genetics. This enabled us to utilize the talent and strengths of both institutions to make this study happen so quickly and successfully.
Our primary outcome was the incremental cost-effectiveness ratio (ICER) — the difference in costs divided by the difference in outcomes — of BRCA1-testing at the time of obstetrical prenatal carrier screening. The secondary outcome measures included BRCA1 mutation positivity rates, cancer diagnoses, cancer deaths, and direct medical costs.
We found that the addition of BRCA1 in prenatal carrier screening was cost-effective with an ICER ratio of $86,000 for every quality-adjusted life year.
— Dr. Shayan Dioun
Key Findings
Dr. Dioun: Our model found that by adding the BRCA1 test to prenatal carrier screening, 3,716 patients with a BRCA1 mutation were identified who otherwise would not have known about their mutation. This subsequently led to the prevention of 1,394 breast and ovarian cancer cases, and 1,084 fewer deaths. We also found that the addition of BRCA1 in prenatal carrier screening was cost-effective, with an ICER ratio of $86,000 for every quality-adjusted life year.
Through cancer screening, we can diagnose cancer in its earliest stages, when the chances of effective treatment are higher. Risk-reducing surgeries, such as mastectomy or removal of the ovaries and fallopian tubes, can prevent cancer altogether and improve survival.
— Dr. Melissa Frey
Future Implications
Dr. Dioun: Our model successfully demonstrated that the addition of BRCA1 testing to obstetrical prenatal carrier screening is a cost-effective management strategy at a time when cancer screening and preventive strategies can make a difference in outcomes.
Dr. Frey: Through cancer screening, we can diagnose cancer in its earliest stages, when the chances of effective treatment are higher. Risk-reducing surgeries, such as mastectomy or removal of the ovaries and fallopian tubes, can prevent cancer altogether and improve survival.
We are currently planning a clinical trial whereby women considering obstetrical carrier screening will have the option to also undergo hereditary cancer screening (including BRCA1 and several other cancer-associated genes). Our primary outcome will be patient acceptability. Furthermore, we will track the results of this testing and try to figure out if this testing translates into cancer prevention and lives saved. We want to see if testing this population prompts women to engage in healthy behaviors, such as going for a mammogram or engaging in other behaviors that reduce their cancer risk.
We’re very excited about the results of this study. This is the type of innovation that's going to make genetic testing for cancer syndromes more accessible and will ultimately lead to more lives saved.




