Addressing CAH Through Comprehensive Care and Research

Dr. Dix P. Poppas
Congenital adrenal hyperplasia (CAH) encompasses a group of autosomal recessive disorders, each of which involves a deficiency of an enzyme critical to the synthesis of cortisol, aldosterone, or both. Deficiency of 21-hydroxylase, resulting from mutations or deletions of the CYP21A gene, is the most common form of CAH, accounting for 90 percent of cases.
Patients with CAH require care and often lifelong treatment, such as is found at the Comprehensive Center for Congenital Adrenal Hyperplasia at NewYork-Presbyterian Komansky Children’s Hospital at NewYork-Presbyterian/Weill Cornell Medical Center. Recognized as the nation’s first Center of Excellence designated by the CARES Foundation (Congenital Adrenal Hyperplasia Research Education and Support), the Center is a major referral site for pediatric and adult patients from around the world.
“For most parents, learning that their child has congenital adrenal hyperplasia is at first shocking news and difficult to absorb,” says Dix P. Poppas, MD, Chief of Pediatric Urology at NewYork-Presbyterian Komansky Children’s Hospital, and the Center’s Surgical Director. “The good news, however, is that the majority of children can go on to lead normal lives with proper diagnosis and treatment.”
CAH affects the production of one or more of three steroid hormones: cortisol, the stress hormone; mineralocorticoids, such as aldosterone, which regulate sodium and potassium levels; or androgens, such as testosterone. In many cases, CAH results in a lack of cortisol and overproduction of androgen, which can cause rapid growth in children or inhibit growth too early. Girls who have too much androgen before birth can develop atypical external genitalia and irregular menstrual cycles, and may have limited sexual function.
The Center’s multidisciplinary team includes pediatric and adult endocrinologists, urologists, surgeons, nurse practitioners, patient care coordinators, dietitians, genetic counselors, reproductive specialists, and psychologists. Karen Lin Su, MD, a pediatric endocrinologist, serves as the Center’s Medical Director. “One of the greatest challenges faced by patients with CAH is locating physicians and other healthcare providers with the expertise needed to deliver their care,” says Dr. Poppas. “We serve a large population of patients with CAH and our team has the skills and experience to meet their healthcare needs throughout their lives.”
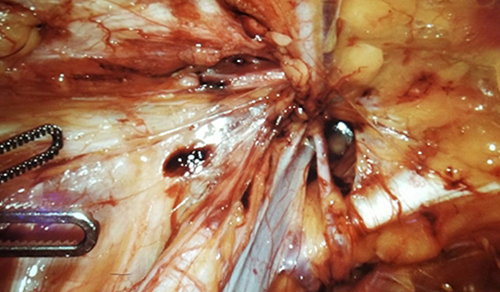
Pediatric endocrinologists often address the underlying hormonal deficiencies caused by CAH through hormone replacement. Treatments involving fludrocortisone, dexamethasone, and hydrocortisone can boost a patient’s cortisone and aldosterone to normal levels. “However, in girls with CAH, the ambiguous genitalia created by the disorder may require corrective surgery in order to allow these children to have normal genital function and appearance,” says Dr. Poppas, who is renowned for his expertise in genital reconstruction, and surgical aspects of disorders of sexual development.
Applying the Zebrafish Model to CAH Genetics
Clinical and basic science research is a key component of the program through the Institute of Pediatric Urology Research Laboratory under the direction of Dr. Poppas at Weill Cornell Medicine. Dr. Poppas’ current research is focused on using the zebrafish model to study the complex genetics of CAH and the use of CRISPR technology to repair the 21-hydroxylase defect.

A zebrafish model is facilitating research on the complex genetics of CAH.
“There is a wide range of consequences in children who have a deficiency of 21-hydroxylase,” explains Dr. Poppas. “In the most extreme form, in utero, where girls have a severe deficiency of 21-hydroxylase, their external genitalia may become virilized. These girls are totally genetic females with normal female internal reproductive anatomy, but on the outside, they can have genital atypia and look more like boys.”
According to Dr. Poppas, children born with CAH have enough of their mother’s circulating hormones initially, but within a day or two after birth, when these hormones are depleted, they can go into adrenal crisis from the cortisol and aldosterone deficiency. Through the efforts of the CARES Foundation, newborn testing for CAH is now being done in every state. “This testing has been very important in identifying children with CAH and has prevented newborn babies from an adrenal crisis and death,” says Dr. Poppas.
Not every patient with CAH has a complete deficiency of enzymes, notes Dr. Poppas. “The genetic mutations that result in CAH are very complicated. Patients can also have a range of 21-hydroxylase enzyme activity from zero percent up to 80 percent. This can affect how the genitalia look, but also how much medication the patient has to take in order to grow normally and prevent adrenal crisis. A person would need less medication and monitoring with activity over 2 percent as compared to less than 2 percent.”
Transition from pediatric to adult care and management of long-term complications from CAH are challenging for both patients and healthcare providers. “Patients are on lifelong medication and must be monitored extensively,” says Dr. Poppas. “If they are in situations that are particularly stressful to the body, for example, if they’re going to have surgery or have a serious infection, they need to change to a stress dose of steroids.”
The ultimate goals for Dr. Poppas’s research are focused on two key areas. One is to activate existing 21-hydroxylase activity to reduce or eliminate the need for mediation; the other is to replace the defective 21-hydroxylase enzyme in patients with CAH with a new, working 21-hydroxylase enzyme. “Based on the 2 percent activity level,” he says, “it might mean we wouldn’t have to replace all of the enzyme activity, but just enough to get over that threshold. If we could do that then there might be a cure for CAH.”

Dr. Yariv Houvras
In their quest to replace the defective 21-hydroxylase enzyme, the laboratory is working closely with Yariv Houvras, MD, PhD, Associate Professor of Research in Surgery at Weill Cornell Medicine, and Jun Yao, a postdoctoral fellow. Using CRISPR genome editing technology, the researchers are deleting the 21-hydroxylase gene in normal zebrafish and examining the consequences. “In the simplest terms, once we’ve knocked out the 21-hydroxylase, we then have to see if we can successfully put it back into the zebrafish,” notes Dr. Poppas. “As I mentioned earlier, there are hundreds of different mutations in the 21-hydroxylase and some of them are known to lead to the classical form of CAH and others to the nonclassical form. At some point, maybe we can model those particular and very specific mutations in 21-hydroxylase. Other ongoing studies are designed to develop chemical screens to discover drugs that might activate 21-hydroxylase activity, which would be an alternative to replacing the defective enzyme.”
The laboratory has been pursuing this first phase of investigation since September 2017 and remains hopeful for a breakthrough. “There is a lot known about other enzymes and pathways in zebrafish, but this is a pathway that’s not as well known,” adds Dr. Poppas. “However, if it were really possible to replace the enzyme activity, that would be a seismic shift in how the lives of patients with CAH would be affected.”
Related Publications

Cutting-Edge Pediatric Robotic Surgery